Liquid Biopsy: How Circulating Tumor DNA Is Changing Cancer Monitoring
 Nov, 23 2025
Nov, 23 2025
For decades, diagnosing and tracking cancer meant drilling into the body to get a tissue sample. A needle through the lung. A cut into the liver. A surgical biopsy after months of waiting. These procedures carried real risks-infection, bleeding, even organ damage-and often couldn’t capture the full picture of a tumor that had changed across different parts of the body. But now, a simple blood draw is replacing many of those invasive steps. That’s the power of liquid biopsy and the circulating tumor DNA, or ctDNA, it detects.
What Is ctDNA and Why Does It Matter?
When cancer cells die, they release fragments of their DNA into the bloodstream. These fragments are called circulating tumor DNA, or ctDNA. Unlike normal DNA floating around in your blood, ctDNA carries the exact genetic mutations that define a person’s cancer. It’s like finding a fingerprint left behind by the tumor itself.
Before liquid biopsy became practical, doctors relied on tissue biopsies to find these mutations. But a single tissue sample might miss key changes if the tumor is made up of different cell types-something called tumor heterogeneity. A biopsy from one part of a lung tumor might show an EGFR mutation, while another part has a KRAS mutation. Liquid biopsy pulls DNA from all over the body, giving a more complete snapshot.
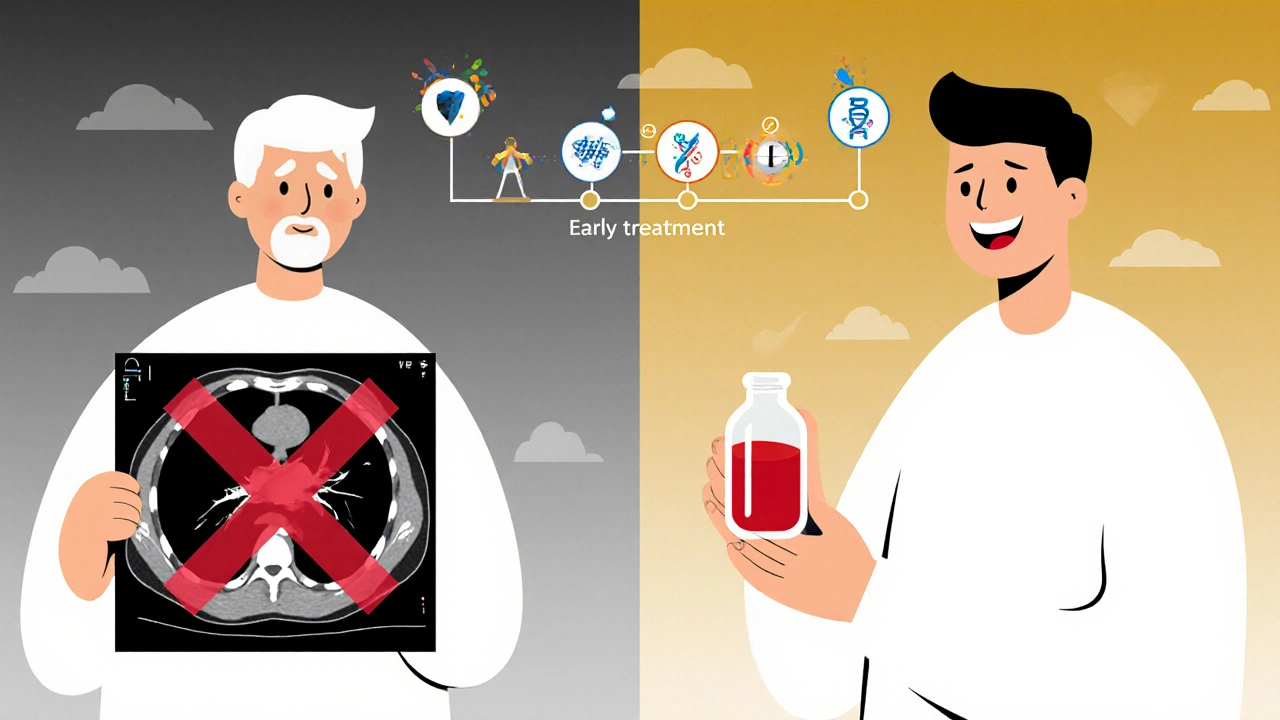
ctDNA isn’t just useful for diagnosis. It’s now the go-to tool for tracking how a tumor responds to treatment. If the levels of ctDNA drop after chemotherapy, it’s a strong sign the drugs are working. If they rise again-even before a scan shows tumor growth-it signals resistance is developing. That’s a game-changer. It means doctors can switch treatments months earlier than waiting for a scan to confirm progression.
How Is ctDNA Detected? The Tech Behind the Blood Test
Detecting ctDNA isn’t easy. It’s like finding one specific grain of sand on a beach. In a single milliliter of blood, there might be billions of normal DNA fragments and only a few hundred from the tumor. That’s why the technology has to be incredibly precise.
There are three main methods used today:
- Digital droplet PCR (ddPCR): This method can spot one mutated DNA molecule among 10,000 normal ones. It’s fast, cheap, and great for tracking known mutations-like EGFR or BRAF-that doctors are already looking for.
- Next-generation sequencing (NGS): This looks at hundreds of cancer-related genes at once. It’s used when the tumor’s mutations aren’t known yet, or when doctors want to find new ones that might be driving resistance.
- Methylation and fragmentomics analysis: This is the newest frontier. Cancer DNA doesn’t just have different mutations-it also has different chemical tags (methylation) and breaks at different lengths than normal DNA. Combining these signals can boost detection rates by 20-30%, especially in early-stage cancers where ctDNA levels are extremely low.
Some labs now use nanopore sequencing, which reads DNA strands in real time, even very short fragments. This helps catch ctDNA that older methods might miss. The key is not just detecting the DNA, but understanding what it means. A mutation found in blood could be from the tumor-or from a harmless age-related change in blood cells, called clonal hematopoiesis. That’s why experienced labs cross-check results with patient history and other data.

Where Liquid Biopsy Shines: Real-World Uses
It’s not a magic wand. But in specific situations, liquid biopsy is already changing outcomes.
1. When tissue isn’t enough
About 20-30% of patients don’t have enough tissue from their original biopsy to test for all possible mutations. In non-small cell lung cancer, where targeted therapies depend on knowing your tumor’s exact genetic profile, liquid biopsy is now recommended by major guidelines as a backup. One study found it identified targetable mutations in 92% of cases where tissue failed.
2. Monitoring treatment in real time
In metastatic breast or colorectal cancer, doctors now test ctDNA every 4-8 weeks during active treatment. If levels stay low, they keep going. If they rise, they switch drugs-often before the patient even feels worse. This has cut down on unnecessary scans and delayed treatment changes.
3. Finding hidden cancer after surgery
After removing a tumor, many patients are told they’re “cancer-free.” But 30-50% of them will relapse. Liquid biopsy can detect ctDNA in the blood months before a scan shows anything. In one study, patients with detectable ctDNA after colon cancer surgery were 85-90% more likely to relapse. That means doctors can offer extra chemo to those who need it-and spare others the side effects.
4. Catching resistance early
A patient on a targeted lung cancer drug might seem to be doing well. But in their blood, a new mutation called T790M appears. That’s the signal the cancer is evolving to escape the drug. With liquid biopsy, doctors can switch to a next-generation drug like osimertinib before the tumor grows. That’s a 3-6 month advantage over waiting for symptoms or scans.
Where It Falls Short: The Limits of Blood Tests
Liquid biopsy isn’t perfect-and it shouldn’t replace all tissue biopsies.
For early-stage cancers (Stage I), ctDNA detection rates are only 50-70%. That’s because small tumors don’t shed much DNA. In cancers like prostate or brain tumors, shedding is even lower. Some patients with advanced cancer still test negative. That doesn’t mean they’re cured-it just means the test missed it.
False positives are another issue. About 10-15% of people over 65 have harmless mutations in their blood cells that look like cancer mutations. These are called clonal hematopoiesis. If a lab doesn’t filter them out, a patient might be told they have cancer when they don’t. That’s why interpreting results requires expertise-not just a machine.
And then there’s cost. A full NGS liquid biopsy can run $1,000-$2,500. Insurance doesn’t always cover it, especially for early-stage disease or screening. Community clinics still struggle to offer it, while major cancer centers have made it routine.
The Future: What’s Next for ctDNA?
The next five years will bring big changes.
Multi-analyte tests are coming. Instead of just looking at DNA mutations, labs will combine ctDNA with methylation patterns, fragment sizes, and even tumor-educated platelets. One study showed this approach could detect Stage I cancers with over 95% accuracy-something no single method can do today.
Artificial intelligence is being trained to spot patterns in ctDNA fragmentation. Cancer DNA breaks in specific ways depending on which organ it came from. AI can learn those patterns and predict not just that cancer is present, but where it started-even if the original tumor is gone.
Regulators are catching up. The FDA has approved 12 liquid biopsy tests since 2020, including Guardant360 CDx and FoundationOne Liquid CDx. These are now used as companion diagnostics-meaning they’re officially tied to specific drugs.
And guidelines are shifting. ASCO and NCCN now recommend liquid biopsy for initial testing in advanced lung cancer when tissue is unavailable. More hospitals are adding it to standard care. By 2030, the global market for liquid biopsy is expected to hit nearly $20 billion.
The goal isn’t to replace tissue biopsy entirely. It’s to make cancer care less invasive, more precise, and faster. Imagine a world where a routine blood test tells your doctor whether your treatment is working-before you even feel sick. That’s not science fiction. It’s happening now.
What Should Patients Know?
If you’re undergoing treatment for advanced cancer-especially lung, colorectal, or breast cancer-ask your oncologist if liquid biopsy is right for you. It’s not for everyone, but if you’ve had a negative tissue biopsy, or if your cancer is hard to reach, it’s a powerful tool.
Don’t assume a negative result means you’re clear. If your doctor says your ctDNA is undetectable, ask: “What’s the sensitivity of this test? Could I still have cancer even if it didn’t show up?”
And if you’re in remission, ask whether ctDNA monitoring is available for surveillance. Detecting recurrence early can mean the difference between a few extra months of chemo-and a chance at long-term control.
Liquid biopsy isn’t the end of cancer diagnosis. But it’s the beginning of a new era-where monitoring happens not in the operating room, but in the waiting room, with a single vial of blood.
Is liquid biopsy better than a tissue biopsy?
Liquid biopsy is not always better-it’s better for different things. Tissue biopsy gives you the full structure of the tumor and is still needed for initial diagnosis. Liquid biopsy is better for repeated monitoring, capturing tumor diversity, and when tissue is unavailable. Many patients now get both: tissue for diagnosis, blood for tracking.
Can liquid biopsy detect cancer early?
It can, but not reliably yet. For Stage I cancers, detection rates are only 50-70%, meaning half the time it misses early tumors. However, new methods using methylation and DNA fragmentation are improving this. Some trials are now testing liquid biopsy for population screening in high-risk groups, like smokers or those with hereditary cancer syndromes. It’s promising, but not ready for general use.
How often should ctDNA be tested?
During active treatment, every 4-8 weeks is typical. After treatment ends, testing every 3-6 months helps catch recurrence early. For some cancers like colorectal, testing every 3 months during surveillance has been shown to detect relapse up to 11 months before scans. Frequency depends on cancer type, stage, and treatment plan.
What if my liquid biopsy shows a mutation I’ve never heard of?
That’s called a variant of unknown significance (VUS). It happens in 15-20% of tests. It doesn’t mean you have a new cancer-it means the lab found a change whose meaning isn’t clear yet. Your doctor may wait for more data, check if it’s in a known cancer gene, or compare it to your tissue biopsy. Don’t panic. Most VUS turn out to be harmless.
Is liquid biopsy covered by insurance?
It depends. Most insurers cover it for advanced non-small cell lung cancer, colorectal cancer, and breast cancer when tissue is insufficient or for monitoring treatment. For early-stage disease or screening, coverage is rare. Always check with your provider and ask if the test is FDA-approved and listed in NCCN guidelines-those are key for approval.
Can liquid biopsy replace imaging scans like CT or PET?
No-not yet. Scans show where tumors are physically located and how big they are. Liquid biopsy tells you what’s happening genetically. They work best together. A rising ctDNA level might prompt a scan to find the new tumor. A shrinking tumor on scan with rising ctDNA might mean the cancer is changing. Using both gives the full picture.
Jacob McConaghy
November 23, 2025 AT 15:46Man, I remember when getting a biopsy meant being hooked up to machines for hours and praying you didn’t bleed out. Now I just get a blood draw and my doc tells me if the drugs are working before I even feel weird. Liquid biopsy feels like sci-fi made real. 🤯
Natashia Luu
November 25, 2025 AT 14:18This article is dangerously oversimplified. The notion that liquid biopsy can replace tissue sampling is not only misleading-it is medically irresponsible. The precision of histopathology cannot be replicated by circulating fragments. This is not progress; it is premature adoption driven by marketing.
akhilesh jha
November 26, 2025 AT 08:21Interesting. In India, access to these tests is still limited to big cities. But I wonder-how many patients in rural areas are being told 'no mutation found' when the test just couldn't detect it? The gap between tech and access worries me. We need cheaper, simpler versions-not just fancier machines.
Jeff Hicken
November 26, 2025 AT 17:55so like… this blood test can like, tell if ur cancer is back?? cool i guess. but why do they charge like 2k for it? my insurance said no. also i think they said my dna was weird but not cancer? idk. my doc just shrugged. lol.
Vineeta Puri
November 28, 2025 AT 16:56While the technological advancements in ctDNA detection are remarkable, it is imperative that clinicians remain vigilant in interpreting results. The presence of clonal hematopoiesis, particularly in elderly patients, necessitates a multidisciplinary approach. We must avoid overdiagnosis and the psychological burden it imposes on patients who are falsely labeled as high-risk.
Victoria Stanley
November 30, 2025 AT 00:55Just had my first liquid biopsy after chemo. My ctDNA dropped 90% in 4 weeks. My oncologist said it’s the best sign possible. I’m not crying because I’m relieved-I’m crying because I finally feel like my body’s being listened to. No needles, no waiting. Just a vial and hope. Thank you, science.
Andy Louis-Charles
November 30, 2025 AT 23:16AI reading DNA fragmentation patterns? 🤖🧠 That’s wild. I saw a paper last week where they trained a model to guess lung vs. colon cancer just from how the DNA breaks. Like, the DNA has a ‘signature’ based on where it came from. We’re basically decoding the tumor’s last scream.
Douglas cardoza
December 2, 2025 AT 16:08My cousin got a negative liquid biopsy after surgery, then relapsed 3 months later. So… what’s the point if it misses half the time? Feels like a gamble. Why not just do the scan and be done with it?
Adam Hainsfurther
December 4, 2025 AT 04:03Just read the part about methylation and fragmentomics. That’s the real breakthrough. DNA isn’t just about mutations-it’s about how it’s folded, tagged, and chopped. It’s like the tumor leaves a fingerprint in how it breaks. We’re not just reading genes anymore. We’re reading the story behind them.
Rachael Gallagher
December 4, 2025 AT 16:06Another American tech fantasy. Meanwhile, real people can’t afford insulin. You’re celebrating a $2,000 blood test while families choose between rent and chemo. This isn’t progress-it’s privilege dressed up as science.
steven patiño palacio
December 5, 2025 AT 02:36For patients with advanced non-small cell lung cancer, liquid biopsy is now standard of care per NCCN guidelines when tissue is insufficient. It’s not experimental-it’s evidence-based. The key is using it appropriately: as a complement to imaging and tissue, not a replacement. Misuse creates harm; proper use saves lives.